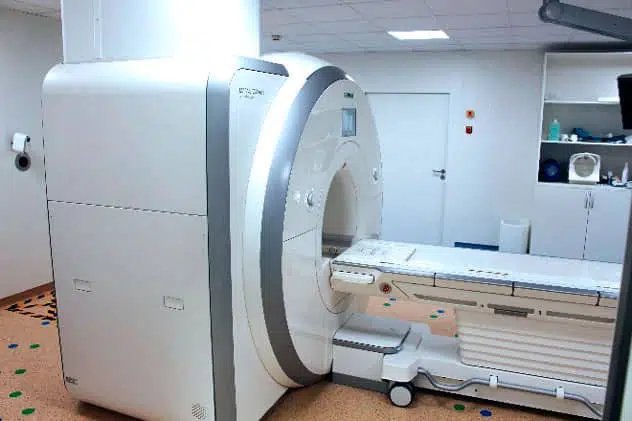
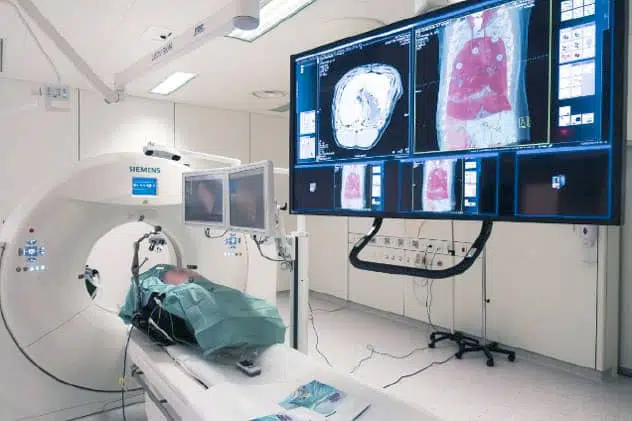
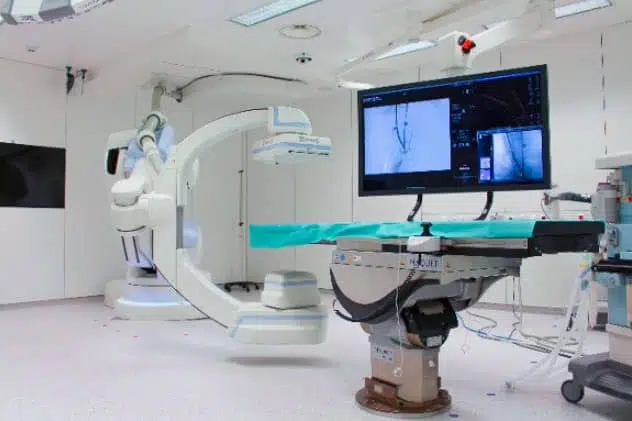
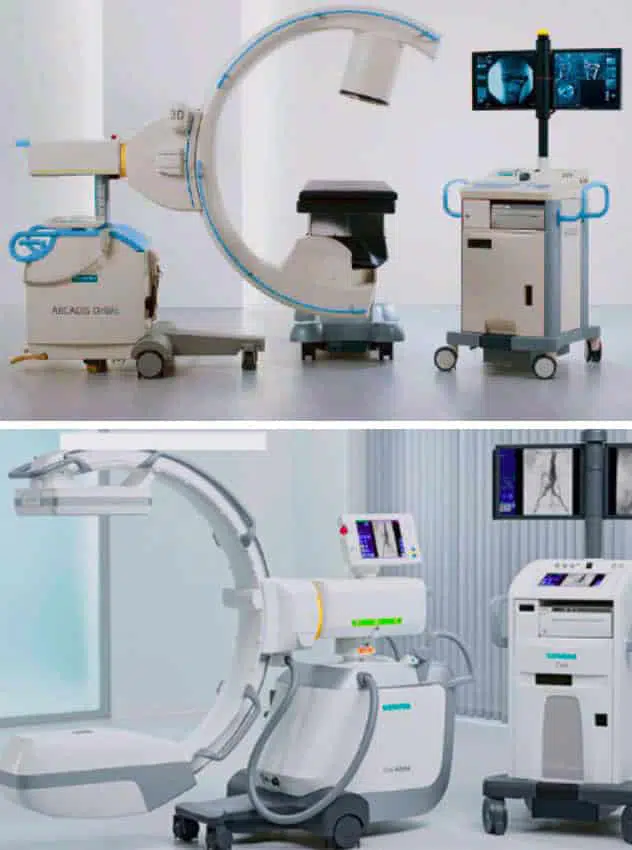
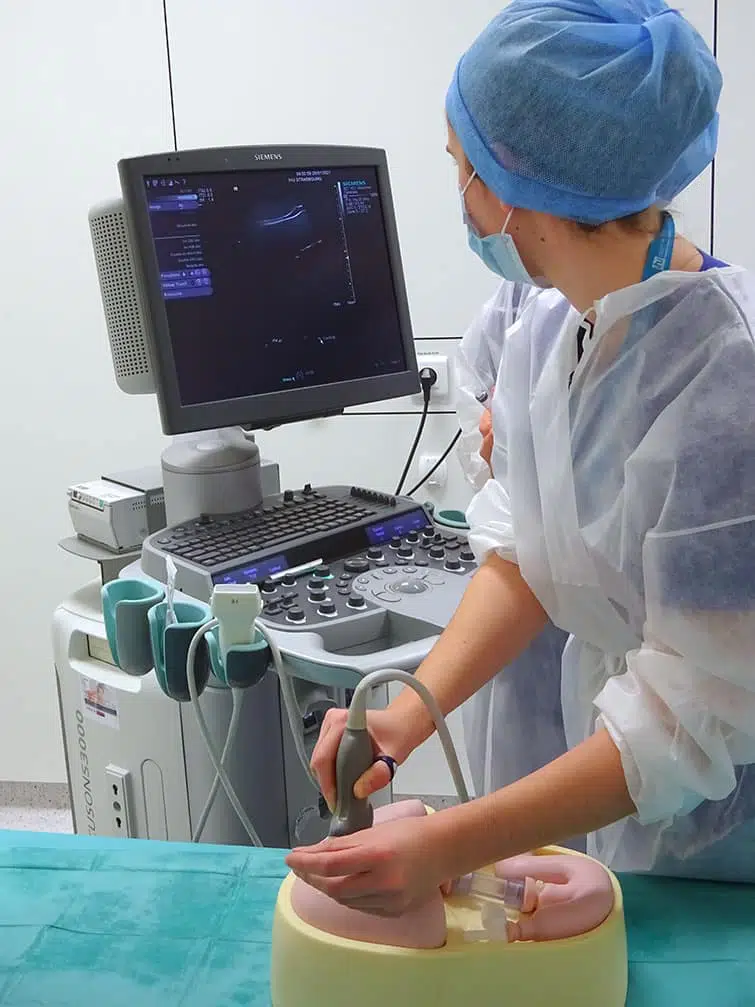
The IHU has a unique preclinical experimental platform dedicated to research, innovation and teaching in image-guided minimally invasive surgery. It features imaging equipment similar to that used in human clinics for diagnostic purposes.
A UNIQUE HUB FOR PRECLINICAL INNOVATION.
The experimental platform
Comprising 5 operating theatres, including 3 “hybrid” theatres combining diagnostic imaging with surgery, the platform is designed to develop innovative minimally invasive approaches. It is a unique place for research, training and innovation in the development and evaluation of medical devices and new image-guided operating procedures.
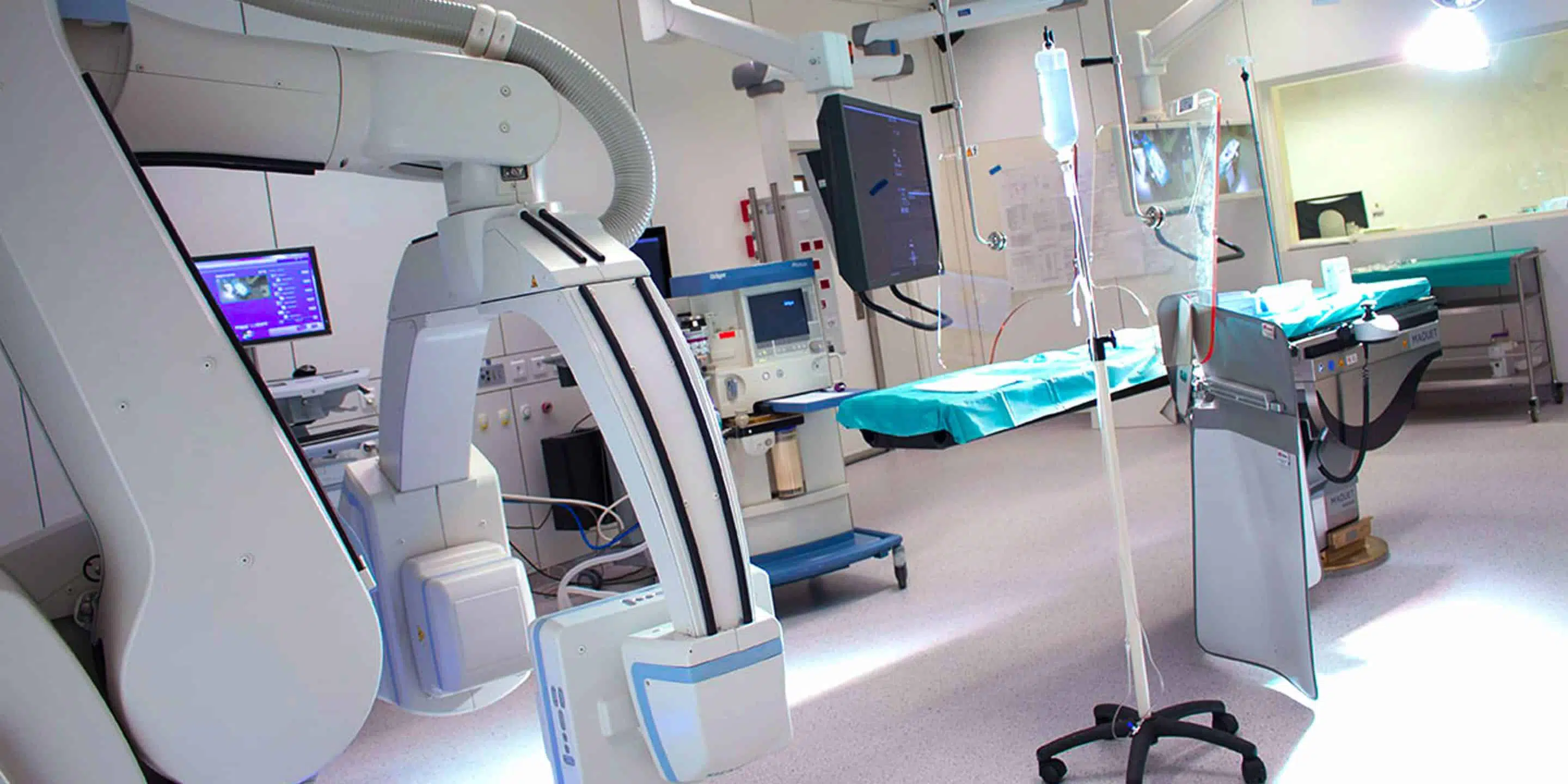
Our equipment
A robotic Cone Beam Computerized Tomography (CBCT) volumetric imaging system (ARTIS ZEEGO – SIEMENS)
In addition to these medical imaging devices, our platform also has: two laparoscopy systems, two endoscopy systems with gastroscopes, duodenoscopes, colonoscopes, and an echo-endoscopysystem.
For full details of our services please see our brochure (in English) at the bottom of the page.

Preclinical research activity
Our network of experts makes this preclinical activity possible, and the expertise they provide is a guarantee of the quality and success of the studies and training carried out at the IHU. The activity is carried out by a qualified support team made up of medical electroradiology manipulators, animal care staff, a biomedical engineer, a project manager and an IT team.
Research and Innovation
The experimental platform enables trials to be carried out on preclinical models for internally funded IHU development projects, as well as for collaborative projects with public and private partners. The studies conducted at the IHU relate to developming new surgical approaches, medical device prototype testing, and assessing artificial intelligence-based software developed for surgery.
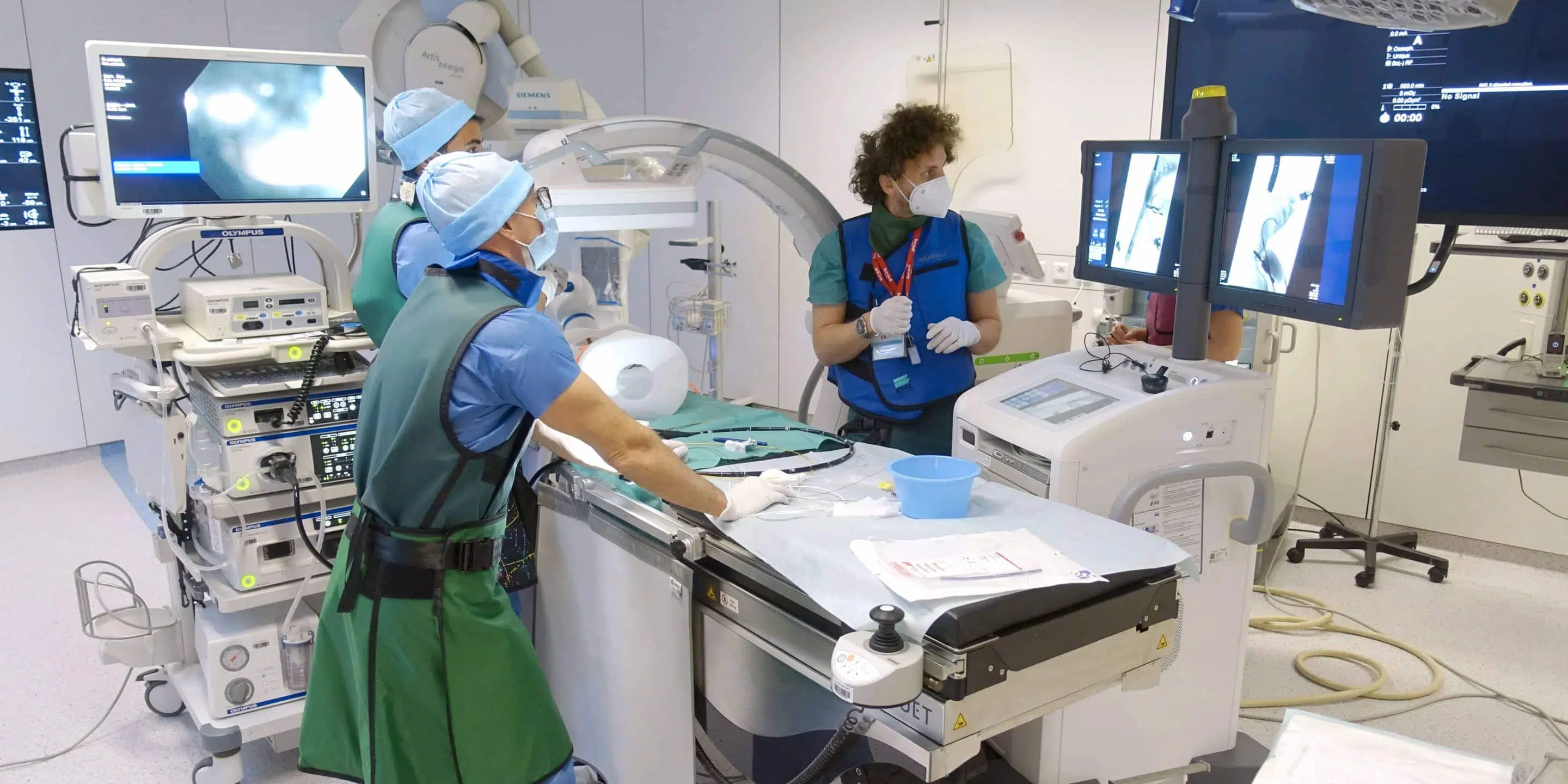
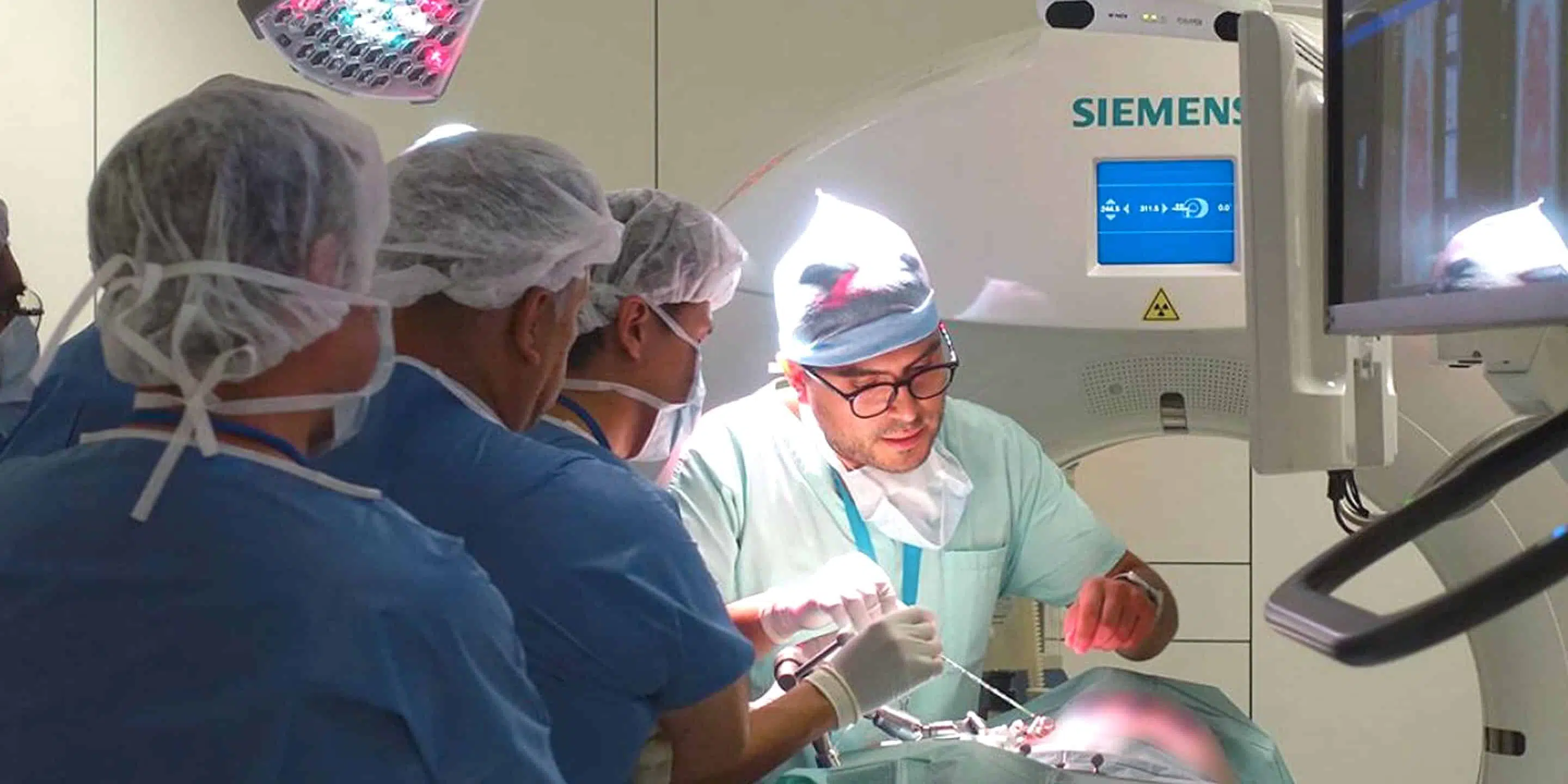
Training and Education
The experimental platform also serves as a dedicated teaching facility for practical sessions in the various university, diploma, technical, industrial and new technology training courses organised by the IHU. These courses take advantage of our wide range of imaging equipment to offer high-quality practical sessions in conditions that mimic the human clinic.
Service offerings
The IHU is the first French testing facility to comply with the principles of Good Laboratory Practice (GLP) for the non-clinical evaluation of medical devices (OECD and FDA regulations). GLP compliance is a regulatory requirement for the conduct of non-clinical safety studies in support of applications for clinical trial authorisation or marketing authorisation for drugs or medical devices.
The “GLP” reference is the result of a European consensus, following the recommendations of the OECD. Its aim is to enable international recognition of data and to guarantee data reliability and quality. This compliance makes it possible to participate in regulatory studies intended for submission to the authorities, and therefore to be a partner of choice for manufacturers or R&D organisations involved in the development of medical devices.
We offer tailor-made services to meet our customers’ needs. We support them from the development of their study plan, advising them on the most appropriate experiments to meet their objectives, right through to providing them with a full report that can include a dossier for submission to the regulatory authorities.
Our experts are involved in these studies to contribute their knowledge and know-how, and can help to improve the product or procedure thanks to their feedback.
Our core business is viceral and digestive surgery, but our network of experts enables us to provide services in all areas of surgery.
The services we offer also apply to the organisation of training sessions with manufacturers to train practitioners in the use of a new medical device or a new therapeutic approach. In particular, we have a catalogue of preclinical in vivo and ex vivo models that we can adapt to the needs of our customers (catalogue provided on request) in order to offer a tailor-made course, adapted to the specific features of the device.