HUS Technical sequencing at IHU Strasbourg: a world first in neoplastic diseases
At the IHU Strasbourg, Prof. Anne Olland and Prof. Pierre-Emmanuel Falcoz, of the Thoracic Surgery and Lung Transplant Department at the University Hospitals in Strasbourg, performed a ground-breaking technical sequencing on Thursday 26 October, benefiting from the institute’s specialty in image-guided minimally invasive robot-assisted surgery.
For the first time, the technical sequencing took place entirely on site, around the patient, who did not need to be transported from one examination to the next. This sequence enabled precise minimally invasive (robot-assisted surgery) and parenchymal sparing (controlled segmentectomy). This surgery offers greater precision and reliability in the early stages of neoplastic disease. The world-first sequencing also expands the IHU Strasbourg’s specialty domains, historically linked to digestive surgery.
This work was the fruit of close multidisciplinary collaboration with IBODEs, radio manipulators, intensive care anaesthetists, as well as interventional radiologists, management teams and industry representatives.
Innovation
The offer of precise, reliable surgery on early-stage neoplastic disease: imaging as close as possible to the surgical procedure enables accurate 3-dimensional reconstruction on a CT scan performed on a patient who is already asleep and in the operating position at the time of surgery, with no positional shift and no lag in the evolution of the disease with a CT scan performed on the same day (compared with a CT scan usually performed lying down, with a lag of one to several days or even weeks in relation to the surgical procedure). The technical sequence takes place around the patient, who does not need to be transported from one examination to the next, enabling precise, minimally invasive, parenchymal-sparing surgery. This technical development remains linked to the HUS’s cross-disciplinary lung cancer research programs.






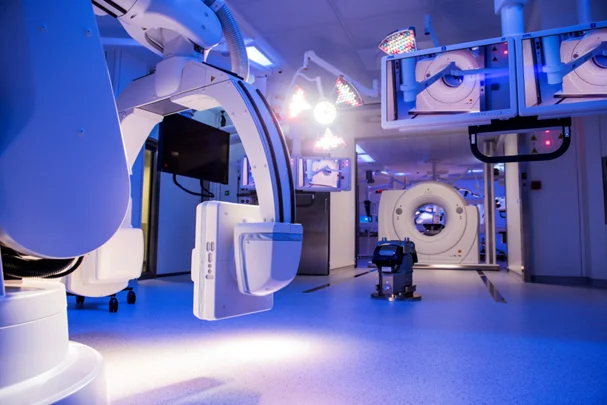
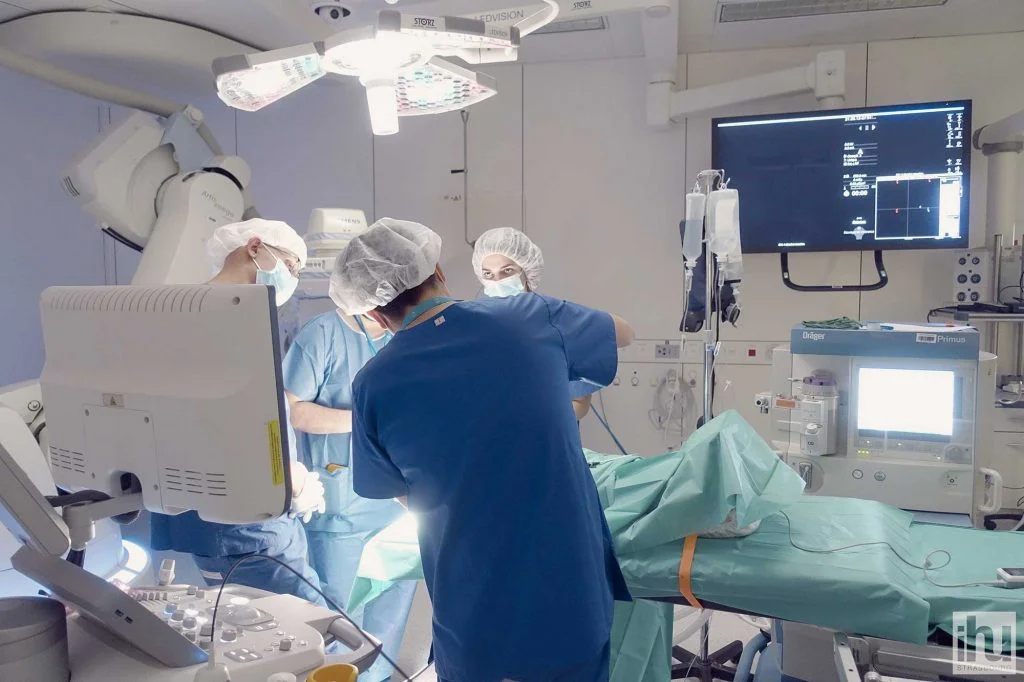 Operating room of the experimental platform at the IHU Strasbourg © IHU Strasbourg
Operating room of the experimental platform at the IHU Strasbourg © IHU Strasbourg The IHU’s experimental platform is fitted with cutting-edge equipment in the fields of surgery, imaging, and minimally invasive therapies (flexible endoscopy, endovascular or percutaneous treatments, robot-assisted surgery). Since its creation, over fifty industry or academic laboratory professionals have been able to work in this platform in a wide range of fields, from hepato-gastroenterology, to cardiovascular studies, neurology and neurosurgery, as well as urology, interventional pneumology, thoracic surgery, gynaecology, and orthopaedics. By coming to the IHU in Strasbourg, manufacturers benefit from the expertise and tailor-made services of an internationally recognized research institute to test and verify the safety and expected effectiveness of their medical devices.
The IHU’s experimental platform is fitted with cutting-edge equipment in the fields of surgery, imaging, and minimally invasive therapies (flexible endoscopy, endovascular or percutaneous treatments, robot-assisted surgery). Since its creation, over fifty industry or academic laboratory professionals have been able to work in this platform in a wide range of fields, from hepato-gastroenterology, to cardiovascular studies, neurology and neurosurgery, as well as urology, interventional pneumology, thoracic surgery, gynaecology, and orthopaedics. By coming to the IHU in Strasbourg, manufacturers benefit from the expertise and tailor-made services of an internationally recognized research institute to test and verify the safety and expected effectiveness of their medical devices.


Recent Comments