The University Hospital Institute (IHU) of Strasbourg has become the first French facility to obtain recognition of compliance with good laboratory practice for the preclinical evaluation of medical devices.
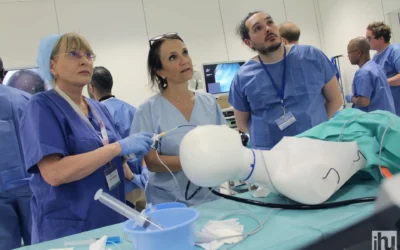
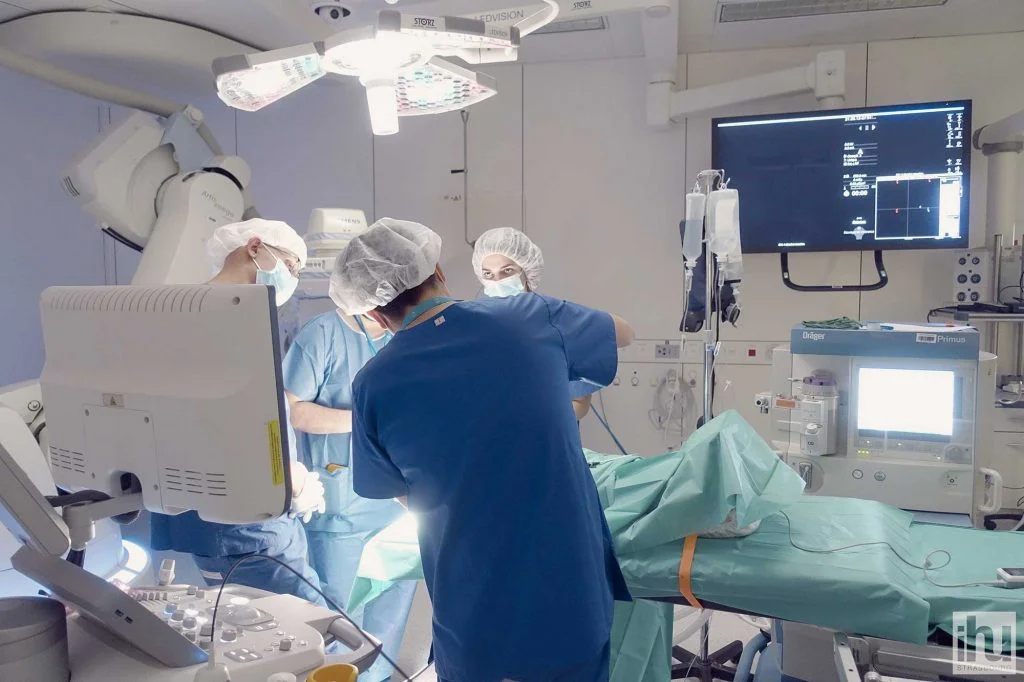 Operating room of the experimental platform at the IHU Strasbourg © IHU Strasbourg
Operating room of the experimental platform at the IHU Strasbourg © IHU Strasbourg
Recognition by the ANSM
On October 21, following over two years of work, the French National Agency for the Safety of Medicines and Health Products (ANSM) awarded the Image-Guided Surgery Institute of Strasbourg recognition of compliance with Good Laboratory Practices. This recognition concerns implantable medical devices (vascular or digestive stents, surgical clips, prostheses) and diagnostic or treatment assistance devices, both hardware and software, including systems based on Artificial Intelligence.
The IHU in Strasbourg is the first academic institute in France, and one of the very first in Europe to obtain this recognition.
A guarantee for Industry
Certification of GLP compliance sets the IHU of Strasbourg apart from its peers for industry partners and collaborators. It allows them to simplify and accelerate their own accreditation processes, but also to test products before they are approved. Valid on the European market (CE marking), but also in all OECD member countries including the USA (FDA) and Japan, the accreditation represents the Industry’s confidence in trusting the IHU’s preclinical expertise.
An experimental platform and network of experts that are unique in the world
According to IHU Director, Benoît Gallix, “The recognition of GLP compliance should enable us to double this contracted research for industry professionals within two years”.
Customized services to meet the needs of the market
We provide tailor-made services to meet the needs of our customers. We accompany them from the preparation of their research plan by advising them on the experiments most suited to meeting their objectives and go as far as providing them with a complete report that can include a file for submissions to regulatory authorities.
Our experts are personally involved in these studies so as to contribute their knowledge and expertise and may also participate in the improvement of the product or the procedure thanks to their experience feedback.
The services we offer also apply to the organization of training sessions with industrial customers to train practitioners in the use of a new medical device or a new therapeutic approach. More particularly, we have a catalogue of preclinical in vivo and ex vivo models that we can adapt to the needs of our customers (catalogue provided on request) for a tailor-made course, adapted to the specificities of the device.