IHU Strasbourg: A Confirmed Commitment to Good Laboratory Practices (GLP)
IHU Strasbourg announces the renewal of its GLP compliance for the evaluation of medical devices, following the inspection conducted by the ANSM (French National Agency for Medicines and Health Products Safety) in October 2025. The institute first obtained this compliance in 2021—an unprecedented achievement for a research center—then renewed it in 2023 and now again in 2025. This recognition reaffirms the institute’s ability to conduct preclinical studies that meet the highest standards for medical device evaluation.
Good Laboratory Practices: A Key Framework for Non-Clinical Evaluation
GLP compliance, defined according to OECD (Organisation for Economic Co-operation and Development) and FDA (U.S. Food and Drug Administration) guidelines, ensures the quality, traceability, and reliability of scientific data. It is essential for supporting clinical trial applications and market authorization submissions for medical devices.
With this renewed compliance, IHU Strasbourg strengthens its position as an internationally recognized key partner for companies aiming to bring innovative medical devices to market.
Tailored to Industry Needs, Backed by IHU Expertise
The IHU team supports its partners throughout every stage of their projects:
-
Study plan design
-
Development of state-of-the-art preclinical models
-
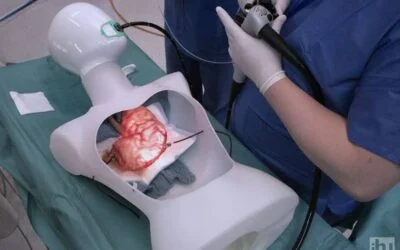
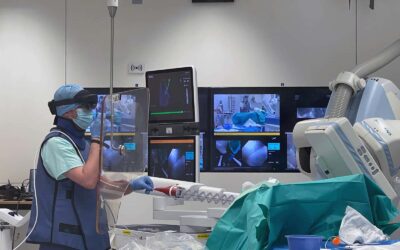
Execution of experiments on a world-unique image-guided surgery platform
-
Production of final reports suitable for regulatory submissions
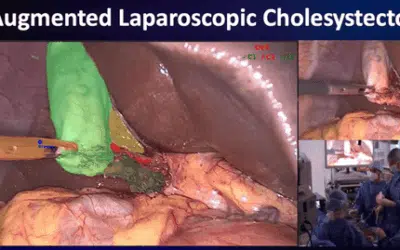
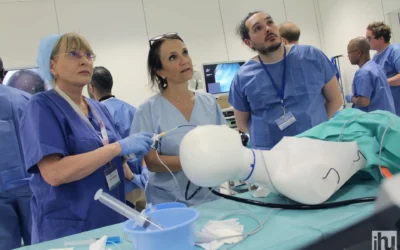
IHU Strasbourg operates across all fields of image-guided minimally invasive surgery through a multidisciplinary network of experts—surgeons, endoscopists, and interventional radiologists—and also offers customized training sessions tailored to the deployment of innovative medical devices.
A Contract Research Offering Powered by IHU’s Scientific Excellence
This renewed GLP compliance illustrates IHU Strasbourg’s ongoing commitment to quality and scientific excellence in minimally invasive, image-guided surgery enhanced by artificial intelligence and robotics. It consolidates the institute’s role as a preferred partner for medical device companies as well as research laboratories seeking to transfer innovative technologies.
For any inquiries regarding contract research at IHU, please contact:
Amélie GRESSIER, Preclinical activities Manager
amelie.gressier@ihu-strasbourg.eu
IHU Strasbourg is a partner in the European TEF-HEALTH consortium (Testing and Experimentation Facility for Health) dedicated to AI- and robotics-based medical devices.